Liposomal technology is a relatively new concept for many complementary healthcare practitioners despite their discovery in the 1960s by Alec D. Bangham.1,2 Since then they have been studied extensively and have been adopted as an advanced phospholipid-based delivery system for nutrients and drugs, and are now the most commonly used nanocarrier.1,3 The unique properties of liposomes, including high biocompatibility and biodegradability, low immunogenicity, non-toxic properties, and the ability to protect and transport both hydrophobic (fat-soluble) and hydrophilic (water-soluble) molecules through the GI tract (overcoming breakdown and metabolism) allows liposomes to deliver their cargo directly to cells.1-4
What are liposomes?
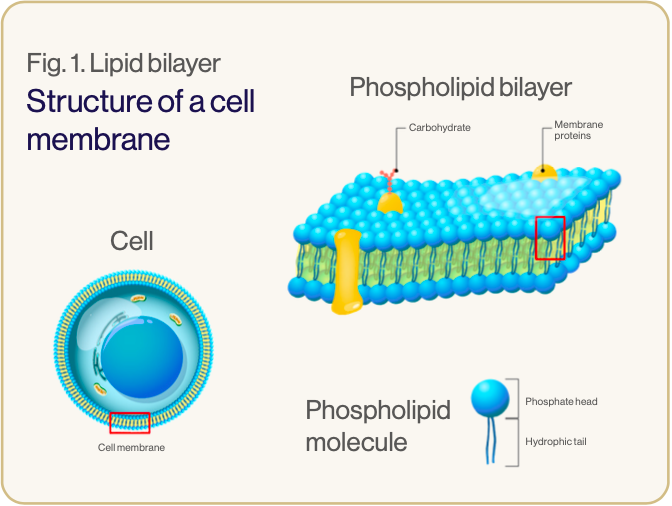
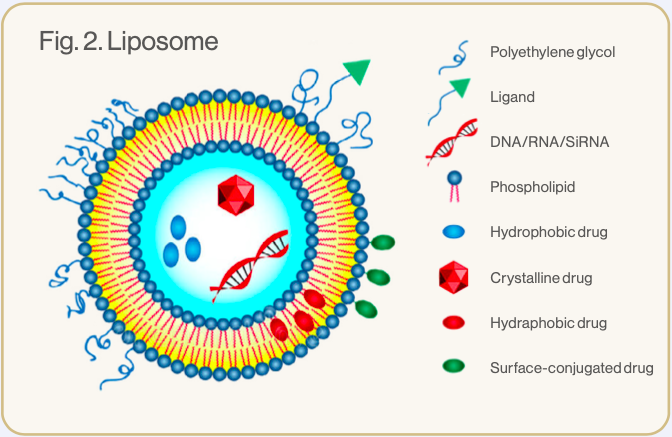
Liposomes are nano-sized vesicles made up of one or more phospholipid bilayers.3 Phospholipids are amphiphilic – they have hydrophilic heads and hydrophobic tails (see image 1). In liposomes the hydrophilic heads face inwards to an aqueous core and outwards to the watery environment of the body, while the hydrophobic tails are positioned within the phospholipid bilayer.1,5 This formation creates a spherical configuration, allowing the liposome to carry and protect hydrophilic elements within its watery core and hydrophobic elements within the lipid bilayer (see image 2).3,5
However, there are many other physiochemical characteristics of liposomes that are important for its function and efficacy as a nanocarrier.3
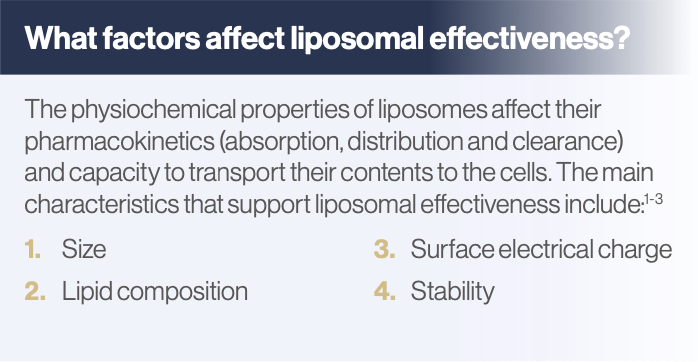
Liposomal size
Liposomes can be up to 1000 nanometers (nm) in diameter particle size, but for greater absorption (including transmucosal) and to cross the blood brain barrier, they need to be between 1 to 100 nm. Liposomes this size are called small unilamellar vesicles (SUVs) – unilamellar meaning ‘with one lipid bilayer’.1-3,5,6
Although they are very small, SUVs have a large surface area and internal core allowing for greater cellular interaction potential and a high encapsulation capacity for hydrophilic elements.2,3 SUVs are more readily transported across the cell membrane and have a high cellular uptake of their cargo. Due to their capacity to adsorb less proteins (opsonins) that trigger the immune clearance by the reticuloendothelial system (RES) involving monocytes and macrophages in the lymph nodes, liver and spleen, SUV’s stay in circulation for longer than larger multilamellar liposomes.1,3,7
Lipid composition
Phospholipids are the main biological components of the body’s cellular membranes, and their composition strongly affects liposomal size, fluidity and electrical charge.1,2 Phospholipid-based liposomes closely resemble the cell membrane, enabling greater biocompatibility and more effective cellular interactions.2 Phosphatidylcholine (PC) from sunflower lecithin is often used in liposomes, as longer chain triglycerides are better at maintaining the solubility of their cargo when compared with short chain triglycerides.6,7 Additionally, being acidic, PC creates a negative charge to the liposomal surface providing increased stability and less phagocytic potential by macrophages. The presence of PC in liposomes supports their low clearance rates by the immune system and longer circulation time before cargo release.3
Surface electrical charge
SUV negatively charged liposomes with longerchain triglyceride compositions also have increased lymphatic absorption, allowing the liposomes to avoid first-pass metabolism.6
Stability
Only very stable liposomes will survive the GI tract intact and be absorbed, with the assistance of the epithelial M cells, into the lymphatic vessels. Liposomes need to be highly stable to remain intact and fuse with, or be internalised by cells to allow for the release of their encapsulated nutrients or drugs.3 As natural phospholipid-based liposomes are less stable than synthetic liposomes they require surface modifications to remain intact after ingestion.1
How are liposomes stabilised for oral use?
Liposomes taken orally are subjected to gastric acids, bile salts, intestinal surfactants and digestive enzymes, which can break down the liposome structure and release its contents into the GI tract. Conventional liposomes are unstable in this environment,3 therefore, advances in liposomal technology have produced more stable forms, improving bioavailability, pharmacokinetics and efficacy of liposomes. The most common surface modifier used in manufacturing for the provision of a protective shield for the liposome is a GRAS (generally regarded as safe) polymer called polyethylene glycol (PEG).3
PEGylated liposomes are referred to as ‘stealth’ or ‘sterically stabilised liposomes’ because the PEG modification (addition of surface hydrocarbon chains) reduces liposome opsonisation and consumption by the RES, allowing the liposome to circulate up to 10-fold longer, equating to hours compared to only minutes with conventional lipsosomes.2,7 Lymphatic absorption and transport is also enhanced with PEGylated liposomes.8
A vitamin E-based, water-soluble PEG, D-α-tocopheryl polyethylene glycol succinate (TPGS or tocofersolan) increases liposome bioavailability by offering high protection against pre-systemic metabolism, improving mucoadhesion, and inhibiting intestinal efflux by P-glycoprotein, increasing permeation across epithelial cells.3,6
Why use liposomes for nutrient delivery?
The harsh GI environment can impair the solubility, stability, absorption and overall bioavailability of orally administered nutrients, particularly those with low solubility or susceptibility to first-pass metabolism.3,6 In stabilised liposomes, nutrients are encapsulated within the lipid bilayer or aqueous core depending on their fat or water solubility, where they are protected from chemical and enzymatic degradation. Nutrients are then passively absorbed across the intestinal epithelium, for transportation through the lymphatic system and are finally released at the cells. Research has shown that stabilised liposomal delivery significantly increases accessibility, bioavailability and stability of many nutrients.4,6
DOWNLOAD A FREE COPY OF THIS ARTICLE
References



